Managing critically ill patients with COVID-19 provides the biggest global healthcare challenge of contemporary days.
COVID-19 virus spreads through the respiratory mucosa, causing widespread damage through cytopathic action, with the induction of severe inflammation and pneumonia, which may progress to lethal respiratory failure in severe cases.
Experiences are showing that 10-20% of cases require ICU admission, consequently, with high risks of developing further complications and long recovery times.
Optimal disease management is still being debated and the therapeutic approach still lacks significant evidence. Therapeutic Apheresis may represent an adjuvant therapeutic possibility in this context, providing support in the control of complications from COVID-19.
Insights
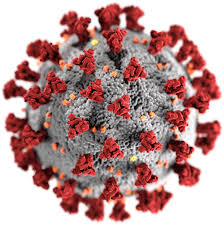
The exacerbated inflammatory response typical of the pathophysiological picture of pneumonia, which is expressed through a “cytokine storm“, well known in literature, seems to be confirmed also in the context of COVID-19, and seems to lead to a “capillary leak syndrome”, leading in the most critical patients to a state of acute respiratory failure, pulmonary edema, and can quickly evolve into a picture of ARDS (Acute Respiratory Distress Syndrome) or sepsis.
In addition, further complications may occur and the clinical picture could evolve to consequences, such as shock, acute renal failure and MOF (Multiple Organ Failure).
Consequently, CytoSorb Therapy, designed to remove cytokines and other circulating mediators from the blood, might represent a control to support and probably prevent the serious complications in critically ill COVID-19 patients.
Experience in Treating Patients with COVID-19 infection
To date, more than 65 critically ill patients with COVID-19 infection have been treated with CytoSorb as supportive therapy for cytokine cascade remodulation in various centers in China, Germany and, above all, Italy.
Although formal clinical data are not yet available due to the extraordinary circumstances, the experience of many Italian centers in recent weeks has allowed to observe encouraging results in more severe COVID-19 patients with severe respiratory syndromes, ARDS and/or multi-organ dysfunction, where the control of the exacerbated inflammatory response seems to have promoted the hemodynamic stabilization of patients and the improvement of respiratory parameters. Modulation of the “cytokine storm” seems to result in endothelial protection, which can be translated into a reduction of the “capillary leak syndrome” and, consequently, in better control of oedema formation and lung infiltration.
The experience in the most serious cases seems to confirm the feasibility of combined treatment with monoclonal anti IL-6 drug, as Tocilizumab, as previously highlighted in the treatment of Cytokine Release Syndrome, contributing synergistically to cytokine reduction. It should be noted that hemoperfusion with CytoSorb can be used in combination with monoclonal therapy, during or after, according to existing protocols in the centers, without interfering with it.
In view of the rationale and the results, albeit preliminary, that have emerged in the recent months in the hospitals where the treatment was introduced, CytoSorb has achieved FDA Emergency Use Authorization for Use in Patients with COVID-19 Infection
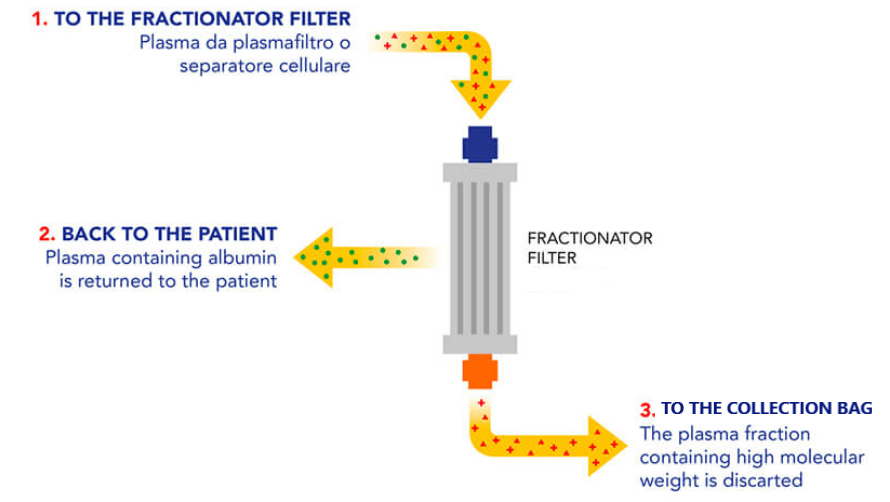
Evidences in literature shows that the use of “convalescent plasma” or “hyperimmune plasma” taken from patients who recovered from COVID-19 can be used to improve the course of critically ill patients in Intensive Care with active disease. This effect appears to be linked to the development of antibodies, in the blood of such patients, against the Sars-CoV-2 virus capable of suppressing virulence.
Double Filtration Plasmapheresis (DFPP) from plasmafilter and Cascade Filtration (CF) from a cell separator, are simple apheretic techniques realized with dedicated equipment, that allow the automatic filtering and concentration of antibodies from the donor’s “convalescent plasma”, with low risk of adverse reactions and loss of “good” components, such as albumin.
In this context, the filtered antibodies can be infused in patients with a still active COVID-19 viral infection.
This procedure, in addition to safeguarding the donor, allows to infuse to the patient a more important quantity of antibodies per unit of infused fluid than a normal “convalescent plasma” donation.
Clinical studies have been approved by the Ethics Committees for the application of this technique is being applied.
U.S. FDA grants CytoSorb emergency use authorization for Use in Patients with COVID-19 infection who are admitted to the intensive care unit (ICU) with confirmed or imminent respiratory failure who have early acute lung injury or acute respiratory distress syndrome (ARDS), severe disease, or life-threatening illness resulting in respiratory failure, septic shock, and/or multiple organ dysfunction or failure. In the Instruction for Use elaborated by FDA, the administration of therapy is as follow:
- Day 1: Change device every 12 hours;
- Day 2: Change device at 24 hours;
- Day 3: Change device at 24 hours;
- Clinical assessment to be made after 72 hours of use to determine if patient is receiving clinical benefit for continuation of therapy
The recent Handbook of COVID-19 Prevention and Treatment from Zhejiang University School of Medicine, China, has recommended blood purification to treat cytokine storm in critical cases of COVID-19 infection.
ClinicalTrials.gov Identifier: NCT04346589
Watch again the registration of the Webinar “Cytokine adsorption in severely ill covid-19 patients – actual scientific results and clinical experiences” held on Thursday, 16th April 2020: https://youtu.be/pSSSZvTQqew
- Brescia Renal Covid Task Force : Alberici F et al., GESTIONE DEL PAZIENTE IN DIALISI E CON TRAPIANTO DI RENE IN CORSO DI INFEZIONE DA CORONAVIRUS COVID-19, published March 18th by the Italian Society of Nephrology on https://sinitaly.org/wpcontent/uploads/2020/03/COVID_guidelines_1703_finale.pdf and by ERAEDTA : https://www.era-edta.org/en/wp-content/uploads/2020/03/COVID_guidelines_finale_engGB.pdf
- Liang T (editor-in-Chief). Handbook of COVID-19 Prevention and Treatment, The First Affiliated Hospital, Zhejiang University School of Medicine, Compiled According to Clinical Experience.
- Chen, Nanshan, et al. “Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.” The Lancet (2020)
- Wang, D, et al. “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirusinfected Pneumonia in Wuhan, China.” JAMA (2020)
- Fei Zhou et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet (2020)
- Claudio Ronco, Paolo Navalesi, Jean Louis Vincent, “Coronavirus epidemic: preparing for extracorporeal organ support in intensive care” www.thelancet.com/respiratory, published online February 6, 2020;
- Bottari G et al. “Multimodal Therapeutic Approach of Cytokine Release Syndrome Developing in a Child Given Chimeric Antigen Receptor- Modified T Cell Infusion”. Crit Care Expl 2020; 2:e0071;
- Long Chen, Jing Xiong, Lei Bao, *Yuan Shi Convalescent plasma as a potential therapy for COVID-19 Lancet Infect Dis 2020 Published Online February 27, 2020 https://doi.org/10.1016/ S14733099(20)30141-9;
The here reported information are non-binding and cannot replace the therapy decisions of the treating physician, who is in all cases responsible for the development and implementation of an adequate diagnostic and therapeutic plan for each individual patient.
Last update: 3/12/2020